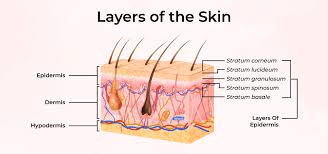
Skin won’t let just anything through. This biological fortress blocks most molecules from entering, and polydeoxyribonucleotide (PDRN) faces an uphill battle because of its size and structure. The average PDRN molecule weighs between 50-1500 kilodaltons, placing it well above the 500 Dalton threshold that skin typically allows to pass through.
Most topical PDRN products deliver disappointing results not because the ingredient fails, but because it never reaches the target tissue. The stratum corneum, that outermost layer of dead skin cells, acts like a brick wall cemented together with lipids. Getting PDRN past this barrier requires specific strategies that address molecular weight, delivery mechanisms, and formulation chemistry. Companies developing PDRN products need to understand these absorption challenges from day one, otherwise they’re selling expensive moisturizers with fancy marketing. For a foundational understanding of this ingredient, see our complete guide to PDRN.
Why PDRN Struggles to Penetrate Skin
The molecular weight problem dominates everything else. PDRN exists as chains of nucleotides, creating large, hydrophilic molecules that can’t easily slip through lipid-rich skin barriers. Smaller molecules (under 500 Daltons) can sometimes squeeze through cellular gaps or dissolve in skin lipids. PDRN can’t do either effectively.
Skin architecture works against large biomolecules on multiple levels. The stratum corneum extends 10-20 micrometers deep with tightly packed corneocytes. Below that sits the viable epidermis, then the dermis where PDRN needs to reach for regenerative effects. Each layer presents different chemical environments. The stratum corneum is lipophilic. The viable epidermis and dermis are hydrophilic. PDRN molecules must navigate both, which they’re not naturally equipped to do.
Charge matters too. PDRN carries negative charges from its phosphate groups, creating electrostatic repulsion with negatively charged components in skin. This ionic interaction further reduces penetration capacity. The molecule basically repels itself away from where it needs to go.
Molecular Weight Reduction Strategies
Breaking PDRN into smaller fragments improves absorption potential, but introduces tradeoffs. Enzymatic hydrolysis can reduce average molecular weight from 1500 kDa down to 50-300 kDa, creating oligonucleotides that retain biological activity while gaining mobility. Studies show fragmented PDRN demonstrates better in vitro penetration through skin models compared to full-length chains.
But here’s the catch. Smaller doesn’t always mean better for efficacy. PDRN works through adenosine receptor activation and growth factor stimulation. Fragment size affects receptor binding and cellular response. Too small, and you lose the beneficial effects. Too large, and nothing penetrates. The sweet spot seems to fall between 100-400 kDa for balancing absorption with activity. For a critical assessment of what research actually shows, see our analysis of PDRN efficacy and the data behind the claims.
Some manufacturers use specific nuclease enzymes to create controlled fragment distributions. This approach produces more consistent molecular weight ranges compared to random hydrolysis. More predictable fragments mean more reliable absorption patterns, though the process costs more and requires careful quality control.
Penetration Enhancers That Actually Work
Chemical penetration enhancers temporarily disrupt the stratum corneum to increase permeability. Effective enhancers for biomacromolecules include several categories worth examining closely.
Lipid-based enhancers:
- Fatty acids (oleic acid, linoleic acid) integrate into stratum corneum lipids and increase fluidity
- Fatty alcohols reduce the organized structure of lipid bilayers
- Ceramide analogs temporarily modify natural lipid composition
These work by making the lipid matrix less rigid. Oleic acid shows particularly strong effects, sometimes increasing permeation of large molecules by 10-fold or more. The mechanism involves creating transient pathways through disrupted lipid organization.
Solvent systems:
- Ethanol and propylene glycol extract lipids and increase hydration
- DMSO (dimethyl sulfoxide) changes protein structure in the stratum corneum
- Transcutol creates a favorable partition environment
Solvents generally work faster than lipid enhancers but may cause more irritation. The balance between efficacy and skin tolerance determines practical application limits.
Surfactants and chelating agents:
- Sodium lauryl sulfate disrupts lipid and protein structures
- EDTA chelates calcium ions critical for maintaining cell-to-cell adhesion
- Polysorbates increase membrane fluidity
Combining enhancers often produces synergistic effects. A formulation using oleic acid plus ethanol plus a surfactant might outperform any single enhancer. However, skin irritation risk scales with penetration enhancement power. Finding the optimal combination requires extensive compatibility testing.

Advanced Delivery Systems
Liposomal encapsulation represents one of the most promising approaches for PDRN delivery. Liposomes are spherical vesicles made from phospholipid bilayers that can encapsulate hydrophilic molecules like PDRN. The lipid structure helps them fuse with skin lipids and deposit contents deeper than surface application.
Deformable liposomes (sometimes called transfersomes) work even better. These vesicles contain edge activators that make membranes ultra-flexible, allowing them to squeeze through pores smaller than their resting diameter. Research demonstrates that transfersomes can carry large molecules across intact skin more effectively than conventional liposomes.
Nanoparticle carriers offer another path. Solid lipid nanoparticles and nanostructured lipid carriers protect PDRN from degradation while improving skin interaction. Particle size matters enormously here. Nanoparticles between 100-300 nanometers show optimal balance between stability and penetration. Smaller particles penetrate better but become unstable. Larger particles stay stable but can’t get through skin effectively.
Microneedle technology bypasses the stratum corneum entirely. Tiny needles (typically 200-800 micrometers long) create temporary microchannels that allow direct PDRN delivery to the viable epidermis and dermis. Dissolving microneedles made from hyaluronic acid or other biocompatible polymers release PDRN as they dissolve in skin tissue. This approach delivers high concentrations exactly where they’re needed, though manufacturing complexity and cost remain barriers to widespread adoption. For realistic expectations on microneedling combinations, see our guide on PDRN and microneedling results.
Formulation pH and Ionic Strength
pH affects everything about PDRN stability and skin penetration. The isoelectric point of PDRN falls around pH 4-5, where the molecule carries minimal net charge. Formulating at or near this pH reduces electrostatic repulsion and potentially improves penetration. But skin pH runs approximately 4.5-5.5, and dramatically shifting this can damage the acid mantle that protects against pathogens.
Buffering systems maintain pH stability during storage and use. Citrate and phosphate buffers work well for PDRN formulations, preventing pH drift that could affect molecule charge distribution. Temperature fluctuations and contamination can shift pH over time, so robust buffering protects product consistency.
Ionic strength modulates how PDRN molecules interact with skin components. High salt concentrations shield charges and reduce electrostatic interactions. Low ionic strength increases charge effects, which can be good or bad depending on the specific formulation goals. Controlled topical delivery systems require careful ionic strength optimization to balance stability with penetration.
Physical Enhancement Methods
Iontophoresis uses electrical current to drive charged molecules through skin. Since PDRN carries negative charges, anodal iontophoresis (negative electrode placement) repels molecules toward and into tissue. Current density, treatment duration, and electrode design all influence delivery efficiency. Clinical studies with other nucleotide-based compounds show iontophoresis can increase penetration by 100-fold compared to passive application.
Sonophoresis applies ultrasound energy to temporarily disrupt the stratum corneum through cavitation effects. Low-frequency ultrasound (20-100 kHz) produces the strongest enhancement, creating acoustic pressure waves that generate bubbles in the coupling medium. Bubble collapse generates localized mechanical forces that open penetration pathways. Treatment protocols typically involve 5-15 minutes of ultrasound exposure before or during PDRN application.
Electroporation delivers short, high-voltage pulses that create temporary pores in cell membranes. This technique excels at delivering large molecules, including nucleic acids and proteins. The electric field causes lipid bilayers to reorganize temporarily, forming aqueous channels. PDRN can transit through these channels if applied during or immediately after pulse delivery. The pores reseal within minutes to hours, trapping delivered molecules in deeper tissue.
Magnetophoresis uses magnetic fields combined with magnetic nanoparticles to guide drug delivery. While less common than other physical methods, this approach shows promise for targeted, controlled release applications. Magnetic PDRN nanoparticle formulations could theoretically be directed to specific skin regions and released on demand.
Combining Multiple Enhancement Strategies
Stacking penetration enhancement methods often produces multiplicative rather than additive effects. A formulation using chemical enhancers plus liposomal encapsulation might achieve 50-100 times better penetration than PDRN in a basic vehicle. Adding physical enhancement like iontophoresis on top of that could push delivery even higher.
But complexity brings challenges. More ingredients mean more potential for incompatibility, instability, and irritation. Overcoming skin barriers for dermatological therapies requires balancing enhancement power with practical formulation constraints.
The order of operations matters significantly. Applying chemical enhancers before physical methods usually works better than the reverse. Pre-treating skin with penetration enhancers for 10-30 minutes before iontophoresis or sonophoresis maximizes the combined effect. Timing intervals between different enhancement steps can dramatically alter outcomes.
Measuring and Validating Bioavailability
In vitro penetration testing uses donated human skin or synthetic membranes mounted in Franz diffusion cells. These experiments measure how much PDRN crosses the barrier over time under controlled conditions. Results provide comparative data between formulations but don’t perfectly predict in vivo performance.
Tape stripping quantifies PDRN distribution across skin layers. Sequential tape strips remove stratum corneum layers, allowing analysis of drug concentration at different depths. This technique works on living subjects and provides depth-resolved penetration profiles. However, it only assesses epidermis penetration, not dermal delivery.
Microdialysis probes inserted into dermis can sample PDRN concentrations in real time. This invasive technique provides the gold standard for measuring actual tissue bioavailability in living subjects. The method’s complexity limits its use mainly to research settings rather than routine product testing.
Confocal microscopy with fluorescently labeled PDRN enables direct visualization of molecule distribution in skin tissue. This approach reveals not just how much penetrates, but where it goes and how it distributes spatially. The technique requires specialized equipment and expertise but generates uniquely valuable data about penetration mechanisms.
Future Directions in PDRN Delivery
Cell-penetrating peptides conjugated to PDRN could guide molecules through biological barriers more effectively. These short amino acid sequences naturally cross cell membranes and might pull attached PDRN along. Early research with other nucleotide drugs shows promise, though chemical conjugation must preserve PDRN’s biological activity.
Smart responsive systems that release PDRN in response to specific skin conditions represent another frontier. pH-sensitive carriers could release payload preferentially in acidic environments like inflammation sites. Enzyme-triggered systems might respond to proteases elevated in damaged tissue. These approaches could concentrate PDRN delivery exactly where regeneration is most needed, particularly for applications like treating hyperpigmentation or reversing sun damage.
Personalized formulation based on individual skin characteristics may eventually optimize PDRN absorption for each user. Factors like age, skin thickness, hydration status, and lipid composition affect penetration capacity. Diagnostic testing could identify the best enhancement strategy for each person’s unique barrier properties. Understanding the regulatory landscape for PDRN products also helps practitioners choose appropriate formulations.
Making PDRN Work Topically
Effective topical PDRN delivery isn’t impossible, but it requires deliberate formulation design. Random application of PDRN in standard cosmetic bases delivers minimal results because the molecule can’t overcome skin barriers alone.
Successful products combine molecular optimization with proven enhancement strategies. Fragment PDRN to appropriate molecular weight ranges. Incorporate effective penetration enhancers at optimal concentrations. Consider advanced delivery systems like liposomes or nanoparticles. Potentially add physical enhancement methods for clinical applications.
The technology exists to make topical PDRN work. Implementation separates products that deliver real benefits from expensive placebos. Companies serious about PDRN efficacy invest in proper delivery science rather than just adding the ingredient to basic formulations and hoping for the best. For clinical approaches that bypass absorption challenges entirely, explore our practitioner’s overview of PDRN in aesthetic medicine.